Because of the ongoing Coronavirus Disease 2019 (COVID-19) public health emergency, there is a growing need for transportation of COVID-19 diagnostic samples (e.g., nasal swabs, vials of sputum, and other related items) from the points of sample collection to the labs for analysis. It is the responsibility of the Pipeline and Hazardous Materials Safety Administration within the U.S. Department of Transportation (USDOT/PHMSA) to ensure the safe transportation of all hazardous materials (HazMat) to, from, or through the U.S. The purpose of this article is to identify and explain the two available methods for the transportation of COVID-19 diagnostic samples.
Note: This will require consideration of the transportation methods recommended by USDOT/PHMSA in its Safety and Advisory Notice for the Transportation of COVID-19 Diagnostic Samples. |
Before we begin…
- 49 CFR 173.134(a)(1): Division 6.2 Infectious Substance means a material known or reasonably expected to contain a pathogen.
- §173.134(a)(1): A pathogen is a microorganism (including bacteria, viruses, rickettsiae, parasites, fungi) or other agent, such as a proteinaceous infectious particle (prion), that can cause disease in humans or animals.
- §173.134(a)(1)(i): A Category B Infectious Substance is not in a form generally capable of causing permanent disability or life-threatening or fatal disease in otherwise healthy humans or animals when exposure to it occurs.
- §173.134(a)(1)(ii): A Category A Infectious Substance is in a form capable of causing permanent disability or life-threatening or fatal disease in otherwise healthy humans or animals when exposure to it occurs.
- §173.134(a)(2): Biological product means a virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, or analogous product, or arsphenamine or derivative of arsphenamine (or any other trivalent arsenic compound) applicable to the prevention, treatment, or cure of a disease or condition of human beings or animals.
- §173.134(a)(4): Patient specimen means those collected directly from humans or animals and transported for research, diagnosis, investigational activities, or disease treatment or prevention. Patient specimens includes excreta, secreta, blood and its components, tissue and tissue swabs, body parts, and specimens in transport media (e.g., transwabs, culture media, and blood culture bottles).
Classification:

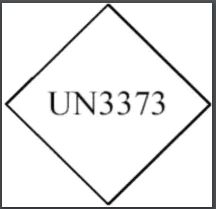
The Centers for Disease Control and Prevention (CDC) directs suspected and confirmed SARS-CoV-2 patient specimens, cultures, or isolates be packed and shipped as UN3373, Biological substance, Category B, Division 6.2 (Infectious Substance).
Scope and Applicability:
- This article can only address the regulations and guidance in place as of the date of its publication. The public and governmental response to the COVID-19 pandemic can change frequently. I will update this article as more information becomes available but please do your own research into the HMR. And, of course, please contact me with any questions.
- This article only considers the domestic regulations of the USDOT/PHMSA for the transportation of HazMat to, from, or through the U.S. It does not include any of the following:
- Dangerous Goods Regulations of the International Air Transport Association (IATA) for the international transportation of dangerous goods by air.
- Dangerous Goods Code of the International Maritime Organization (IMO) for the international transportation of dangerous goods by vessel.
- The regulations of any other country other than the U.S.
- This article will only identify and explain the aspects of the HMR applicable to a COVID-19 diagnostic sample classified – as directed by the CDC – as: UN3373, Biological substance, Category B, Division 6.2 (Infectious Substance).
Interested in site specific training at your site that covers this topic, and more! Ask me about my Onsite Training |
Option 1 for Transportation of COVID-19 Diagnostic Samples:
An option to pack and ship a COVID-19 diagnostic sample can be found at §173.199. If packed and shipped according to the requirements of this section, the diagnostic sample is not subject to any other regulations of the HMR, except:
- HazMat incident reporting at §171.15 and §171.16.
- Cargo location on aircraft per §175.75(b).
Also, if a diagnostic sample meets the definition of a hazard class other than Division 6.2 (e.g., Class 3 Flammable Liquid), it must be packed and shipped in compliance with the applicable regulations in addition to those of this section.
This is the most restrictive option within the HMR for this HazMat. It is also the method outlined in this USDOT/PHMSA poster: Guide to Packaging Category B Diagnostic Samples. And it is the only method described as available for COVID-19 Diagnostic Samples in the USDOT/PHMSA Safety Advisory Notice for the Transportation of COVID-19 Diagnostic Samples. In short: lacking further exploration, you may think the packing instructions of §173.199 are your only option for the transport of COVID-19 diagnostic samples, but they aren’t.
But, to be thorough, I’ll describe the requirements of §173.199 for transportation of COVID-19 diagnostic samples.
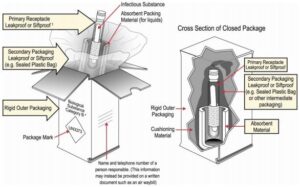
Triple Packaging:
Three layers of packaging are required, to include:
- A primary receptacle that is leakproof for liquids and sift proof for solids.
- The primary receptacle must be packed into a secondary packaging that also is leakproof (for liquids) or sift proof (for solids).
- The primary receptacle must be packed in the secondary packaging so that it cannot break, be punctured, or leak contents into the secondary packaging under normal transport conditions.
- If several fragile primary receptacles are placed in the secondary package, they must be wrapped or separated to prevent contact between them.
- If the diagnostic sample in the primary receptacle is a liquid, absorbent material of sufficient quantity to absorb all liquid – and not compromise the integrity of the cushioning material or outer packaging – must be placed between the primary and secondary receptacle.
- If the diagnostic sample in the primary receptacle is a solid but a residual liquid may be present or the solid may become a liquid in transportation, it must be packed and shipped as if it was a liquid.
- The secondary packaging must then be placed in the third layer of packaging: a rigid outer packaging.
- A specification packaging is not required.
- The completed package must be designed, constructed, maintained, filled, and closed so there is no release of HazMat under normal transportation conditions.
- Package effectiveness must not be substantially reduced due to:
- Minimum and maximum temperatures.
- Changes in humidity and pressure.
- Shocks, loadings, and vibrations normally encountered during transportation.
- The packaging must be capable of successfully passing the drop test as specified in §178.609(d) at a drop height of at least 1.2 meters (3.9 feet). Following the drop test, there must be no leakage from the primary receptacle, which must remain protected by absorbent material, when required, in the secondary packaging.
- At least one surface of the outer packaging must have a minimum dimension of 100 mm by 100 mm (3.9 inches).
Like this article? Subscribe to my Monthly Newsletter No marketing emails! |
Clear Instructions:
- Clear instructions on filling and closing the packaging must be provided by the packaging manufacturer and subsequent distributors to the person who prepares the package for transport.
- Packagings must be filled and closed in accordance with the information provided by the packaging manufacturer or subsequent distributor.
- A copy or electronic image of these instructions must be retained by the manufacturer and subsequent distributors for at least one year from the date of issuance, and made available for inspection upon request.
No Other HazMat:
A packaging containing inner packagings of Category B infectious substances may not contain other hazardous materials except:
- Refrigerants, such as dry ice or liquid nitrogen. These must comply with §173.199(d).
- Anticoagulants used to stabilize blood or plasma.
- Small quantities of Class 3, Class 8, Class 9, or other materials in Packing Groups II and III used to stabilize or prevent degradation of the sample, provided the quantity of such materials does not exceed 30 mL (1 ounce) or 30 g (1 ounce) in each inner packaging. Such preservatives are not subject to the requirements of the HMR.

If to be transported by air:
No surprise, the packaging requirements are greater if the COVID-19 diagnostic sample is to be transported by air. Remember this article addresses solely the regulations of USDOT/PHMSA which normally aren’t followed when the mode of transport is aircraft. Usually, the Dangerous Goods Regulations of IATA apply to air transportation of HazMat. Pursuant to the HMR:
- For a liquid diagnostic sample the primary or secondary receptacle must be capable of withstanding an internal pressure of not less than 95 kPa (0.95 bar, 14 psi).
- Also for a liquid diagnostic sample, the maximum quantity in each primary receptacle, including any material used to stabilize or prevent degradation of the sample, may not exceed 1 L (34 oz). The maximum quantity in each outer packaging, including any material used to stabilize or prevent degradation of the sample, may not exceed 4 L (1 gallon).
- For a solid diagnostic sample that will remain a solid, the maximum quantity in each outer packaging, including any material used to stabilize or prevent degradation of the sample but not including body parts, organs, or whole bodies, may not exceed 4 kg (8.8 lb). The outer packaging limit does not apply to ice, dry ice, or liquid nitrogen when used to maintain the integrity of the samples.
Each package, overpack, pallet, or unit load device containing a Category B infectious substance must be inspected for leakage when it is unloaded from the aircraft. If evidence of leakage is found, the cargo compartment in which the package, overpack, pallet, or unit load device was transported must be disinfected. Disinfection may be by any means that will make the material released ineffective at transmitting disease.
Hazard Communication:
- The following square-on-point mark must be displayed on the outer packaging on a background of contrasting color. Refer to §173.199(a)(5) for the required specifications for this package mark.
- The name and telephone number of a person who is either knowledgeable about the material being shipped and has comprehensive emergency response and incident mitigation information for the material, or has immediate access to a person who possesses such knowledge and information, must be included on a written document (such as an air waybill or bill of lading) or on the outer packaging. This telephone number must be monitored during a company’s administrative office hours.
Training:
Each person who offers or transports a Category B infectious substance under the provisions of this section must know about the requirements of this section. This does not require full HazMat Employee training.
More Information if Following the Packing Instructions of §173.199:
- USDOT/PHMSA Guide to Packaging Category B Diagnostic Samples
- USDOT/PHMSA guidance: Transporting Infectious Substances Safely
Daniels Training Services, Inc. 815.821.1550 |
Summary of Option 1:
As noted earlier in this article, USDOT/PHMSA guidance documents and the USDOT/PHMSA Safety Advisory Notice for the Transportation of COVID-19 Diagnostic Samples seem to provide only one option for the transportation of COVID-19 diagnostic samples, and that is the one described in option 1.
However, a close reading of these documents reveals that the packing instructions of §173.199 are not the only ones available for a COVID-19 diagnostic sample. Another one – a better one – is available at §173.134(b)(10).
Q: Are you sure? The Safety Advisory Notice only describes the requirements of §173.199; it makes no mention of the packaging exception at §173.134(b)(10). Nor do either of the USDOT/PHMSA guidance documents referred to above. How do you know the packaging exception of §173.134(b)(10) is available for the transportation of COVID-19 diagnostic samples?
A: First of all, while the Safety Advisory Notice only describes the packing of COVID-19 diagnostic samples subject to §173.199, it also includes the following language:
To ensure their safe transportation, SARS-CoV-2 diagnostic samples must be packaged and offered for transportation in conformity with the applicable requirements in the HMR for Category B infectious substances.
This – presumably – includes and certainly does not exclude the packaging exception for these diagnostic samples at §173.134(b)(10).
To clarify the position of USDOT/PHMSA in regards to the transportation of COVID-19 diagnostic samples and the language in the Safety Advisory Notice I contacted the Hazardous Materials Information Center on August 04, 2020. The Hazardous Materials Specialist confirmed that though the language in the Safety Advisory Notice is unclear, COVID-19 diagnostic samples are eligible for the packaging exception at §173.134(b)(10).
Q: A phone call conversation? That’s it?! I’m supposed to ship COVID-19 diagnostic samples in a manner that does not appear in USDOT/PHMSA guidance and all you have is a telephone conversation with an unidentified Specialist?!?
A: At this point yes, but I submitted a request for a letter of interpretation to USDOT/PHMSA the next day (08.05.20), and received an email confirmation just over a week later (08.13.20) informing me of a possible 8 week wait for a reply. A few days after that I received a letter via USPS dated 08.13.20 also confirming receipt and providing a tracking number (20-0062). In a separate telephone conversation on August 12th with the same Hazardous Materials Specialist I was told the letter of interpretation will confirm the position expressed in the initial telephone conversation. Once issued by USDOT/PHMSA, the letter of interpretation will have the force and effect of the HMR. I will update this article as the LOI is issued. Until then you could ship COVID-19 diagnostic samples under the requirements of §173.199 (Option 1) and wait for the LOI or you could take advantage of the packaging exception of §173.134(b)(10) -aka: Option 2 – now.
And here it is!
It took a bit longer than 8 weeks, but on March 09, 2021 I received a response to my request for a letter of interpretation I submitted on August 05, 2020. The answer confirmed my conclusion described above and summarized again here:
- Nothing in the Safety Advisory Notice or elsewhere in the HMR prevent the classification of COVID-19 diagnostic sample as a Category B, Division 6.2 Infectious Substance when offered for transportation.
- COVID-19 diagnostic samples include:
- Nasal swabs
- Vials of sputum
- Other related items
- Classified as a Category B, Division 6.2 Infectious Substance, COVID-19 diagnostic samples are eligible for the exception at §173.134(b)(10). This exception is explained below as Option 2.
Read the letter of interpretation: LOI 20-0062
Contact me with any questions you may have about the transportation of hazardous materials by air, highway, vessel, or rail International and Domestic Daniels Training Services, Inc. 815.821.1550 |
Option 2 for Transportation of COVID-19 Diagnostic Samples:
Let’s walk through it.
49 CFR 173.134 defines a Division 6.2 Infectious Substance and its many types and forms (some of which are included at the beginning of this article). It also includes several packaging exceptions. The definitions are in subparagraph (a), most of the packaging exceptions (with the exception of the one for regulated medical waste) are found in subparagraph (b). 173.134(b) reads:
The following are not subject to the requirements of this subchapter as Division 6.2 materials:
What follows are sub-subparagraphs (1-16) identifying Division 6.2 Infectious Substances not subject to – i.e., excepted from – regulation as a Division 6.2 (and presumed then to be excepted from the remainder of the HMR unless classified as some other type of HazMat) if the conditions of the exception are met.
We will stop at §173.134(b)(10), which reads:
A Division 6.2 material, other than a Category A infectious substance, contained in a patient sample being transported for research, diagnosis, investigational activities, or disease treatment or prevention, or a biological product, when such materials are transported by a private or contract carrier in a motor vehicle used exclusively to transport such materials. Medical or clinical equipment and laboratory products may be transported aboard the same vehicle provided they are properly packaged and secured against exposure or contamination. If the human or animal sample or biological product meets the definition of regulated medical waste in paragraph (a)(5) of this section, it must be offered for transportation and transported in conformance with the appropriate requirements for regulated medical waste.
Now let’s break it down.
“A Division 6.2 material, other than a Category A infectious substance…”
As noted at the beginning of this article, COVID-19 diagnostic samples are classified as a Category B, Division 6.2 (Infectious Substance).

“…being transported for…”
If the Category B, Division 6.2 Infectious Substance is contained in a patient sample (human or animal), the reason for its transportation must be one of the following:
- Research
- Diagnosis
- Investigational activities
- Disease treatment or prevention
Or, it may be a biological product, in which case the above reasons for transportation are not required.
Transportation:
Transportation must be by a private or contract carrier. Neither of these terms are defined in the HMR and the Federal Motor Carrier Safety Administration (FMCSA) no longer distinguishes between a common or a contract motor carrier. Briefly summarized:
- A private carrier is not a “for hire” carrier and does not provide transportation services for a third party. A private carrier uses its own vehicles to transport its own HazMat.
- A contract carrier does provide “for hire” transportation services for a third party, but under continuing contracts with one person or a limited number of persons. Read: FAQ: What is a contract carrier?
Transportation must be in a motor vehicle. That means the mode of transportation must be by highway.
The motor vehicle must be used exclusively to transport the patient sample or biological product. This does not mean that this is all the motor vehicle ever transports. It means at the time the exception is required, the COVID-19 diagnostic samples (with some exceptions, see below) are the only material in transport on the motor vehicle.
Also…
The following may be transported aboard the vehicle at the time of transportation if properly packaged and secured against exposure or contamination:
- Medical or clinical equipment
- Laboratory products
If you like this article, please share it using any of the social media platforms identified at the bottom of this article. |
If a Regulated Medical Waste:
Then it must be shipped according to the appropriate regulations for a regulated medical waste.
Read: FAQ: Are wastes associated with COVID-19 a hazardous waste?
Read: Off-Site Transportation of COVID-19 Waste
And that’s it!
No other requirements of the HMR apply, not even those of Option 1.
Daniels Training Services, Inc. 815.821.1550 |
Conclusion:
As of the writing of this article, both Option 1 and 2 are available for the transportation of COVID-19 diagnostic samples. Perhaps you may prefer to defer the use of Option 2 until the LOI is issued by USDOT/PHMSA. If so, please check back in no later than 8 weeks from August 13, 202o (and perhaps sooner). Or, you may prefer to begin using Option 2 right now. Or, you may stay with Option 1 regardless of the exception at §173.134(b)(10). Whichever you choose, make certain you comply with all applicable regulations of the HMR.